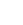2,3-Dimethyl-2,3-Diphenylbutane: Structure and Synthesis
Introduction:
2,3-Dimethyl-2,3-diphenylbutane is an organic compound recognized for its distinct structural arrangement and significance in advanced chemical studies. Its combination of aromatic rings and substituted carbon centers makes it valuable for examining stereochemistry, molecular stability, and reaction behavior. This article provides a detailed overview of its structure, synthesis pathways, and potential applications across research and materials science.
Molecular Structure:
This compound is a symmetrical hydrocarbon with the molecular formula C??H??. It features a butane backbone where the 2 and 3 carbon atoms are each substituted with a phenyl group and a methyl group.
This dense substitution pattern leads to:
- A compact, rigid three-dimensional geometry
- Significant steric hindrance
- Distinct reactivity behavior compared to simpler hydrocarbons
The presence of aromatic rings contributes to electronic stability and limited rotational freedom, which influences how the molecule participates in chemical reactions.
Synthesis:
Several reliable synthetic routes are used to prepare this compound:
1. Alkylation–Reduction–Hydrolysis Route
- Begins with alkylation of phenylacetonitrile using bromopropane
- Produces a substituted nitrile intermediate
- Reduction of the nitrile yields the corresponding butanenitrile
- Final hydrolysis step provides the target hydrocarbon
This method uses accessible starting materials and standard organic reactions.
2. Friedel–Crafts Alkylation Route
- Benzene reacts with 3-methyl-2-bromopropene in the presence of a Lewis acid
- Generates an alkylated aromatic intermediate
- Subsequent reduction and purification yield the final compound
This pathway highlights the role of aromatic substitution in constructing the molecule.
Applications:
The compound's structural features enable several potential uses:
In Organic Synthesis
- Serves as a reference or model molecule for studying reaction mechanisms
- Provides steric control in multi-step synthetic processes
- Helps design molecules with tailored reactivity profiles
In Materials Science
- Its rigid framework and aromatic content make it suitable for exploring new materials
- May contribute to improved strength or stability when incorporated into polymers or composite structures
These attributes make it a valuable candidate for both fundamental and applied research.
Conclusion:
This compound's distinctive molecular design, along with its accessible synthesis routes, makes it an important subject in organic chemistry. Its steric and electronic characteristics offer advantages in both synthetic methodology and material development. Continued research may uncover additional applications and enhance its relevance in scientific fields.